
Company Description
Danapha Pharmaceuticals is a leading pharmaceutical manufacturing and trading company in Vietnam. The company operates two GMP-WHO standard pharmaceutical factories for Eastern medicine and non-beta lactam drugs, along with a new GMP-EU standard factory under construction in the high-tech zone of Đà Nẵng. The work environment is friendly, with a professional work ethic that supports personal development.
Role Description
This is a full-time on-site role for a QA Manager - GMP_EU Factory at Danapha Pharmaceuticals located in Đà Nang. The QA Manager will be responsible for overseeing quality assurance activities in the GMP-EU factory, ensuring compliance with regulatory standards, and implementing quality control measures to maintain product quality and safety on a day-to-day basis.
Qualifications
- Quality Assurance, Regulatory Compliance, and Quality Control skills
- Knowledge of GMP-EU standards and pharmaceutical manufacturing processes
- Experience in conducting quality audits and inspections
- Strong attention to detail and analytical skills
- Excellent written and verbal communication skills
- Ability to work effectively in a team and cross-functional environment
- Bachelor's degree in Pharmacy, Chemistry, or related field
- Certifications in Quality Management or QA/QC would be beneficial

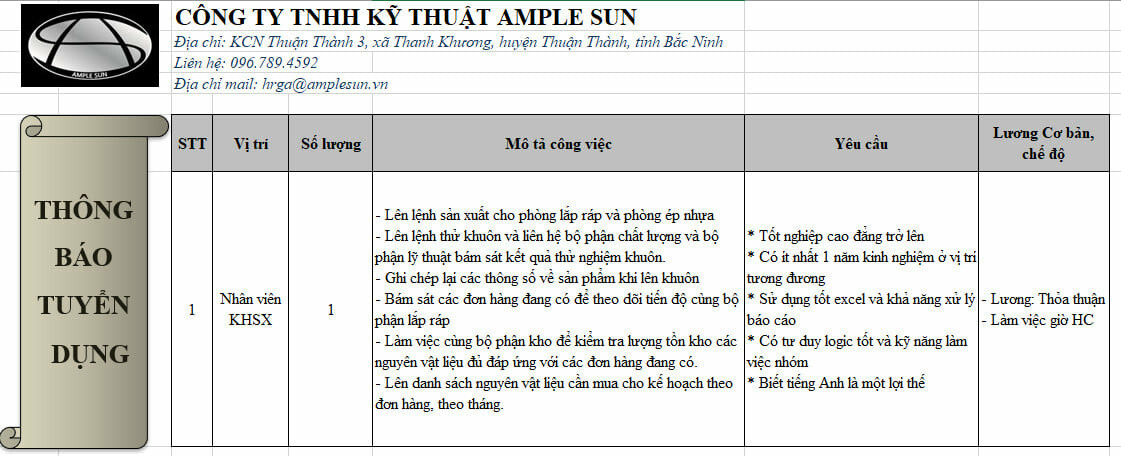
Công ty TNHH Kỹ Thuật Ample Sun
Vốn đầu tư 100% của Italia.
Mã số thuế: 0108043344
Người ĐDPL: Giglio Antonio
Ngày hoạt động: 20/09/2013
Giấy phép kinh doanh: 0312489562
Ngành nghề: Sản xuất lắp ráp linh kiện điện tử viễn thông, dây cáp, sợi quang học.
Bạn làm việc tại Kỹ Thuật Ample Sun? Chia sẻ kinh nghiệm của bạn


 Tweet
Tweet
 Facebook
Facebook
 Copy Link
Copy Link